The FDA just approved the Freestyle easier to use monitor in June of 2010 and received the European CE Mark in May of 2011. In December of 2009 there were strip recalls for the old version of the Freestyle, however other Abbott models had a lot more strips recalled in December of 2010.
Abbott Diabetes Care Recalls Tons of Glucose  Test Strips–The US Has the FDA Recall Blues-Solution Has Been Touted Here for a Year-It’s Time for a Fix–Readers Have Voted
Test Strips–The US Has the FDA Recall Blues-Solution Has Been Touted Here for a Year-It’s Time for a Fix–Readers Have Voted
Supply interruptions was the reason given for the discontinuing in the US; however in Europe they will continue to sell and market, so not enough money here in the US is the first question I ask and glad we have enough other glucose monitors available for diabetics. I just hope glucose monitors don’t take the same path as some of the drugs we can’t get today and hope this is an isolated instance. Abbott still has many other Freestyle monitors they sell and market besides this model, but this one with wireless looked to be pretty advanced. BD
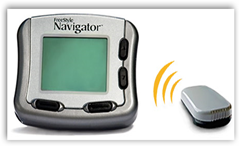
Abbott Laboratories (NYSE:ABT) announced that it permanently discontinued its FreeStyle Navigator glucose monitor due to an inconsistent supply chain that has amounted to several disruptions in product availability for U.S. patients.
"We know FreeStyle Navigator System customers in the U.S. have lived with some uncertainty and frustration," the company wrote in its official statement. "We are grateful for the commitment patients and providers have devoted to the FreeStyle Navigator System over the years, and we are sorry that we have been unable to consistently meet their needs."
"The discontinuation of the FreeStyle Navigator System in the U.S. is not for safety reasons," Abbott wrote. "The System is safe and effective and continues to be available to patients in seven other markets outside the U.S."
http://www.massdevice.com/news/diabetes-abbott-takes-freestyle-glucose-monitor-us-market
Tidak ada komentar:
Posting Komentar